Iron

Iron exists in two different states:
- Ferrous (Fe+2) or iron (II)
- Ferric (Fe+3) or iron (III)
Iron can be converted from ferrous iron (Fe+2) to ferric iron (Fe+3) by removal of an electron known as oxidation. This ability to be converted between different states is why iron is very useful to living organisms. All known forms of life require iron, however, because of its low solubility in water, iron is often a limiting nutrient. Measuring iron levels is important to a wide range of applications and industries including food and beverage production, drinking water, wastewater, agriculture, freshwater and saltwater aquarist, and boilers and cooling towers.
Due to the insolubility of its ferric (Fe+3) form, the methods for measuring iron in water use a reducing agent to convert the iron in a sample to its soluble ferrous (Fe+2) form. The ferrous iron then reacts with a molecule to produce a colored complex, which is used to measure the concentration of iron in the sample. Because of the instability of the ferrous iron, which is easily changed to the ferric form when the solution comes in contact with air, determination of ferrous iron requires special precautions and may need to be done in the field at the time of sample collection. It is also advised to avoid long storage times or exposure of samples to light.
There are two common methods for measuring iron in water, the TPTZ method and phenanthroline method. In the TPTZ method, a reducing agent is first added to the sample to convert iron in the sample to the ferrous (Fe+2) form. Then 2, 4, 6-tripyridyl-s-triazine, or TPTZ, is added, which reacts with ferrous iron (Fe+2) to form a deep blue-purple color. The TPTZ method for total iron has the advantages of being simple, having a high sensitivity, and is free from many common interferences by other atoms and molecules. The TPTZ method is capable of determining iron concentrations as low as 1 µg/L.
The phenanthroline method is adequate for the analysis of natural or treated waters. Iron is brought into solution and reduced to the ferrous state (Fe+2). The solution is then treated with 1,10-phenanthroline (phenanthroline) at pH 3.2 to 3.3. Three phenanthroline molecules then react with one ferrous iron (Fe+2) to form a characteristic orange-colored complex. The intensity of the color development is directly proportional to the amount of ferrous iron (Fe+2). When EPA reporting is necessary, digestion of the sample is also required.
Hanna Instruments offers a variety of technologies to measure iron. Spectrophotometers and benchtop photometers can measure a wide variety of other water quality parameters that are important for water quality. A portable photometer and a Checker HC are dedicated meters to the measurement of iron are also available.
Solutions
Solutions include the CAL Check standards for verifying and, if needed, the calibration of our portable photometers. Each CAL Check standard is supplied with a Certificate of Analysis stating the accuracy and traceability of the standard.
Accessories
Accessories include a COD digestion reactor for use with the total iron reagents that require digestion. Also available are spare cuvettes and caps for photometers.
Chemical Test Kits
Iron chemical test kits are available in a variety of ranges and include a basic color cube with five points of resolution and the Checker disc version that has a continuous scale for increased resolution.
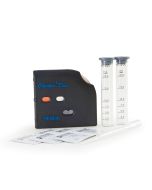
Checker HC
Checker HC are reagent based colorimeters which measures the color change that occurs based on iron concentration in the sample. The handheld colorimeters, like portable and benchtop instrumentation, use the Beer-Lambert principle to determine the color change and then display results on the LCD.
Portable Photometers
Portable photometers dedicated to the measurement of iron are available in two ranges along with multiparameter versions that also measure other key water quality parameters. The portable photometer has a CAL Check feature that allows for performance verification and if needed, recalibration using the CAL Check standards. The portable meter is available as a meter only or as a kit. The kit version includes a rugged carrying case and CAL Check standards.
Benchtop Photometers
Benchtop photometers include multiparameter versions for the laboratory, wastewater, water conditioning and water treatment markets. These meters include many other key water quality parameters. All benchtop photometers have a digital pH electrode input allowing it to be used as a traditional pH meter.
Spectrophotometers
Spectrophotometers are available that have iron methods pre-programmed into the meter. The spectrophotometer offers the highest precision due to the quality of the optical system that has a wavelength accuracy of +/- 1.5 nm. The spectrophotometer allows for custom methods to be developed.
Reagents
The following are the reagents used with the photometers, Checker HC and chemical test kits. The total iron by digestion method can be use on the wastewater benchtop photometer or spectrophotometer.